Medical miracle or notorious lynchpin of misinformation?
Ivermectin has been hailed as a “wonder drug” and, according to the UNESCO World Science Report, a critical component of “one of the most triumphant public health campaigns ever waged in the developing world.”
However, since the onset of the COVID-19 pandemic, the National Institutes of Health (NIH) and affiliated health authorities have vociferously recommended against ivermectin as a potential treatment for the virus.
Though the Food and Drug Administration (FDA) has approved ivermectin for human use in treating conditions caused by parasites, it has also insisted that ivermectin “has not been shown to be safe or effective” when it comes to treating COVID-19.
In a social media message that has gone viral, the FDA labeled it as a drug for horses and not fit for human consumption: “You are not a horse. You are not a cow. Seriously, y’all. Stop it.”
The post made headlines and was one of the FDA’s most successful social media campaigns. Yet, research findings seem to contradict the public health organization’s recommendations.
A growing body of research shows that ivermectin is an essential treatment for COVID-19. Many doctors have praised the drug for its broad yet effective antiparasitic, antiviral, antibacterial, anti-inflammatory, anti-cancer, and autophagic properties.
Ivermectin: Antiparasitic Beginnings
Ivermectin made its name through its significant benefits in treating parasitic infections.
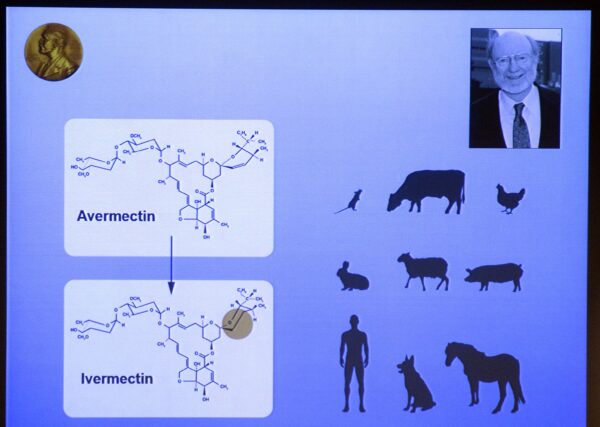
In 1973, Satoshi Omura and William C. Campbell, working with the Kitasato Institute in Tokyo, found an unusual type of Streptomyces bacteria in Japanese soil near a golf course.
In laboratory studies, Omura and Campbell discovered that this Streptomyces bacteria could cure mice infected with the roundworm Heligmosomoides polygyrus. Campbell isolated the bacteria’s active compounds, naming them avermectins, and the bacteria was thus called S. avermitilis.
Despite decades of searching worldwide, researchers have yet to find another microorganism that can produce avermectin.
It was changing one of the bonds of avermectin through a chemical process that produced ivermectin, which was proven successful in treating onchocerciasis and lymphatic filariasis, both of which are debilitating diseases common in the developing world.
Though its broad antiparasitic functions are not well understood, it is known that ivermectin penetrates parasites’ nervous systems, turning off their neurons’ actions, possibly deactivating and killing them.
As part of a donation campaign launched in 1988 by Merck & Co., Inc., the manufacturer of ivermectin, the drug was used in Africa to treat river blindness. Also called onchocerciasis, river blindness is a tropical disease caused by Onchocerca volvulus worms. It is the second-most common cause worldwide of infectious blindness.
The Onchocerca worms mature in the skin of an infected individual (“the host”). After mating, female worms can release into the host’s skin up to 1,000 microfilariae a day; the female worms live for 10 to 14 years. The presence of these worms can lead to scarring in the tissues and, when microfilariae invade the eye, can cause visual impairment or complete loss of vision.
The World Health Organization estimates that 18 million people are infected globally, and 270,000 have been blinded by onchocerciasis.
When Merck distributed ivermectin in areas hardest hit by the disease, treatment benefited the residents’ overall health and led to economic recovery. Ivermectin replaced previous drugs that had devastating side effects.
“Ivermectin proved to be virtually purpose-built to combat Onchocerciasis,” Omura wrote in a study he co-authored in 2011.
Ivermectin has also proven effective against lymphatic filariasis, known as elephantiasis. Parasitic worms transmitted through the bite of an infected mosquito can grow and develop in lymphatic vessels, which regulate the body’s water balance. When certain vessels are blocked, the areas—typically the legs and genitals—can swell, with the legs enlarging to elephant-like stumps.
Worldwide, more than 120 million people are infected, 40 million of whom are seriously incapacitated and disfigured.
The World Health Organization listed ivermectin as an essential drug and has advised many countries to run annual campaigns to rid people of these parasites. Such recommendations are a solid testament to ivermectin’s safety.
For their work, including the discovery of avermectin, in 2015, Omura and Campbell were among three recipients of the Nobel Prize in Physiology or Medicine.
It is an indispensable drug for the underdeveloped world, with about 3.7 billion doses administered as part of global campaigns during the past 30 years. To this day, ivermectin remains a staple drug of tropical areas and an essential drug in treating onchocerciasis, lymphatic filariasis, strongyloidiasis, and scabies.
Ivermectin and COVID-19
Analyses of studies on ivermectin have found it effective as a prevention, a treatment for acute COVID-19, and in advanced stages of infection by the virus.
1. Ivermectin as a Prophylaxis
Prophylaxis intervenes in the first phases of COVID-19 infection, which is mainly asymptomatic, when the virus replicates to increase its viral load—symptom onset occurs after the viral load peaks.
Ivermectin can be effective in the early stages of infection. Outside the cells, ivermectin can attach to parts of the virus, immobilizing it and preventing it from entering and infecting human cells.
Ivermectin can also enter the cell to prevent the virus from replicating. SARS-CoV-2 needs cell replication machinery to make more of the virus; ivermectin attaches and blocks a protein critical to this process, preventing viral production.
Additionally, ivermectin can be absorbed from the skin and stored in fat cells for a long time.
“Because it’s lipid soluble, it is stored and slowly released, [so] once you’ve taken a prophylactic dose, and I think it’s like the cumulative dose of about 400mg, that your risk of getting COVID is close to zero and you can actually stop it for a while,” said Dr. Paul Marik, a widely published critical care specialist with 500 peer-reviewed papers to his name, in an interview with The Epoch Times.

Marik co-founded the Front Line COVID-19 Critical Care Alliance (FLCCC), a group of physicians formed in the early days of the pandemic and dedicated to treating COVID-19. According to interviews, many of the group’s doctors have successfully treated COVID-19 with ivermectin. The organization’s other co-founder, Dr. Pierre Kory, has written a book about ivermectin’s use and controversy during the pandemic.
Dr. Sabine Hazan, a gastroenterologist with 22 years of experience in clinical research, told The Epoch Times that she would advise ivermectin use for only a short time in critical patients rather than recommending the use of it as a prophylaxis.
Continuous use of ivermectin—as with all drugs—can make the body dependent on the drug rather than working to fix itself.
2. Ivermectin for Early and Acute COVID
Many peer-reviewed studies have found that ivermectin, when used by itself or in conjunction with other therapies in symptomatic patients, reduces ventilation time, time for recovery, and the risk of progressing to severe disease. (pdf 1, pdf 2, pdf 3)
This is likely due to ivermectin’s anti-inflammatory role in multiple pathways, achieved by clearing out the viral particles by immobilizing them, reducing inflammation, and improving mitochondrial action.
Suppose the early viral replication is not controlled and cleared out soon enough by the body’s immune system. In that case, the infection can become severe or even hyperinflammatory, possibly leading to systemic organ failures.
Ivermectin can also directly interact with immune pathways, suppressing inflammation and reducing the chances of developing a cytokine storm. A cytokine storm occurs when the immune system is hyperactive and hyperinflammatory. Though ivermectin can help to clear out the virus and its particles, the inflammatory state of the tissues and the organs can often cause more damage than the virus itself.
Ivermectin also likely improves gut health, which plays an essential role in immunity by preventing bacteria and viruses from infecting people via the gut.
In a published study, Hazan hypothesized that ivermectin helps COVID-19 patients by increasing the levels of Bifidobacteria—a beneficial bacteria—in the gut.
As the CEO and founder of her own genetic sequencing research laboratory, ProgenaBiome, Hazan noticed that the Bifidobacteria levels in her stools would increase after she took ivermectin. Critical COVID patients would have “zero Bifidobacteria,” which can often be a sign of poor health.
In her peer-reviewed study on hypoxic patients, she observed that COVID patients with low oxygen levels from the cytokine storms in their lungs would improve within hours of administering ivermectin.
“When people die of COVID, they die from the cytokines—they couldn’t breathe anymore. It’s almost like an anaphylactic reaction. So when you give them ivermectin at the moment they’re about to crash, you’re boosting the Bifidobacteria [and increasing their oxygen],” Hazan said.
She explained that ivermectin is a fermented product of Streptomyces bacteria. Streptomyces are within the same group Bifidobacteria are from, which may explain why ivermectin temporarily boosts Bifidobacteria.
Ivermectin also helps with mitochondrial function. During severe COVID-19, patients often experience pulmonary dysfunctions due to lung inflammation, reducing oxygen flow. This can cause stress to the mitochondria, leading to fatigue, and, when severe, may cause cell and tissue death. Ivermectin has been shown to increase energy production, indicating that it is beneficial to the mitochondria.
Furthermore, ivermectin can bind to the spike protein—a distinctive structural feature of the COVID virus which has a crucial role in its pathogenesis. In systemic disease, the spike protein can enter the bloodstream and bind to red blood cells to form blood clots. Ivermectin can prevent blood clots from forming in the body.
3. Ivermectin for Long COVID and Post-Vaccine Symptoms
The number of studies supporting ivermectin to treat long COVID and post-COVID-19 vaccine symptoms is limited. However, doctors treating these conditions have observed successful results with ivermectin.
An Argentinian study published in March 2021 is the only peer-reviewed study evaluating ivermectin for long COVID.
Researchers found that in patients reporting long COVID symptoms—including coughing, brain fog, headaches, and fatigue—ivermectin alleviated their symptoms.
Mechanistically, ivermectin can improve autophagy. This process is usually switched off during COVID-19 infections. By switching autophagy back on, ivermectin can help cells clear remnant viral proteins out, returning stability to the cell.
Like acute and severe COVID-19, chronic spike protein triggers inflammation, and ivermectin can reduce such responses by suppressing inflammatory pathways and lessening the damage to tissues and blood vessels.
The Changing Public Health Messaging on Ivermectin
The NIH’s stance on ivermectin has changed several times.
Early in the pandemic, there was little information on ivermectin as a potential treatment for the virus.
The first study that mentioned ivermectin as a potential COVID-19 treatment came from Australia in April 2020. Researchers administered ivermectin to SARS-CoV-2-infected monkey kidney cells in the laboratory and found the drug beneficial in very high doses. However, the researchers concluded that further study was needed. Many health agencies, including the NIH, the CDC, and other global health regulators concluded that ivermectin could kill the virus only at toxic levels.
Even now, NIH’s statement on ivermectin for COVID-19 reads: “Ivermectin has been shown to inhibit replication of SARS-CoV-2 in cell cultures. However, pharmacokinetic and pharmacodynamic studies suggest that achieving the plasma concentrations necessary for the antiviral efficacy detected in vitro would require administration of doses up to 100-fold higher than those approved for use in humans.”
In October 2020, the first clinical study showing the benefits of ivermectin was published by the journal CHEST. The study found ivermectin to reduce mortality rates in COVID-19 patients and garnered immediate attention.
The study’s lead author, Dr. Jean-Jacques Rajter, is a critical care doctor specializing in pulmonary medicine.
Rajter gave a testimony (pdf) of his findings to the Senate Committee on Homeland Security & Governmental Affairs in December 2020.
The day after he saw the Australian study, one of his COVID patients dramatically deteriorated from breathing normally at room oxygen levels to requiring intubation. The patient’s son pleaded with Rajter to save his mother using whatever options available. Rajter recognized that hydroxychloroquine would be ineffective in the advanced stages of COVID. After much deliberation, he tried ivermectin.
“The patient deteriorated as expected for about 12 more hours but stabilized by 24 hours and improved by 48 hours. After this, two more patients had similar issues and were treated with the ivermectin-based protocol. Based on experience, these patients should have done poorly, yet they all survived,” the testimony read.
More clinical studies were published, showing the benefits of ivermectin as a prophylactic treatment. (pdf 1, pdf 2).
The findings encouraged the use of ivermectin among doctors desperate to find a cure.
Meanwhile, by October 2020, research into COVID-19 vaccines and the use of remdesivir to treat the virus was already in full swing.
According to the FDA, specific criteria should be met for the EUA (Emergency Use Authorization) to be granted for vaccines and medications, including that there are “no adequate, approved, and available alternatives.”
Some doctors say that if ivermectin’s use for COVID had been approved, it would have made the EUAs for vaccines and remdesivir null and void.
Following the Australian study, the FDA published a statement, “FAQ: COVID-19 and Ivermectin Intended for Animals,” highlighting the use of ivermectin in animals and advising against the use of ivermectin for COVID-19.
The NIH also discouraged the use of ivermectin, albeit briefly. On Jan. 14, 2021, the NIH changed its statement, writing that there was no evidence to recommend or disapprove the use of ivermectin. However, in April 2022, the statement changed to strongly disapproving of using ivermectin.
“We [Marik, Kory, and Dr. Andrew Hill, a virologist and consultant to the WHO] had a conference with NIH in January of 2021. We presented our data, and Andrew Hill presented the data he had done…there were a number of studies at that point, which were very positive,” said Marik.
Health Authority Overreach
Despite the NIH’s neutral statement on ivermectin for most of 2021, the FDA actively campaigned against using ivermectin in COVID-19 patients. On Aug. 26, 2021, the CDC sent an emergency warning against using ivermectin; a few weeks later, the American Medical Association and affiliated associations called for an end to ivermectin use.
Many doctors were thus discouraged from using ivermectin, and pharmacies refused to prescribe it. State health agencies warned against using ivermectin, and medical boards removed the medical licenses of doctors who prescribed ivermectin, alleging misinformation.
Yet using the FDA’s statement against ivermectin to ban its use in COVID-19 cases would be considered an overreach. Since the FDA approved ivermectin in 1996, this made the drug acceptable for off-label use.
“The fact that it’s not FDA approved for COVID is irrelevant because the FDA endorses the use of off-label drugs at the clinician’s discretion,” said Marik.
As an ironic side effect of the messaging on ivermectin, people suddenly found themselves unable to access ivermectin, and some turned to veterinary-grade ivermectin.
Though veterinary ivermectin is the same product as medicinal ivermectin, the manufacturing standard is not the same as it is for human-grade pharmaceuticals.
Contradictory Research and Campaigns
Though the initial research in 2020 showed promising results for ivermectin, published studies reported conflicting findings by the following year.
The NIH has funded many studies on the effectiveness of ivermectin, the most recent being ACTIV-6.
Individuals can participate in the study once they develop COVID by selecting ivermectin from four other drugs. The drug was sent to them via mail. This method means that some people in the study could have recovered by the time they received the ivermectin.
There are some controversies regarding this study.
The first is that the authors changed the primary endpoints during the study, which is heavily frowned upon as it can affect the validity and reliability of the outcome.
Initially, the primary endpoint was the number of deaths, hospitalizations, and symptoms reported at day 14.
This was changed to the number of deaths, hospitalizations, and symptoms by day 28. In the actual published study, there was another change, with the endpoint being duration of COVID-19 symptoms.
A rapid review published by the Massachusetts Institute of Technology (MIT) implied that the endpoints were changed because, by the time the study commenced, there were far fewer events of death and hospitalizations; as a result, there would not be enough data for a reliable comparison.
Indeed, the data at the ACTIV-6 livestream showed that the ivermectin group reported only one death; this death would not be considered relevant to the research because the patient was hospitalized and died before he took ivermectin.
There were also further implementations in the study that could impact the observed effectiveness of the drug.
On average, this study’s participants received treatment six days after first reporting symptoms. Patients needed to report eligible symptoms and test positive for COVID-19 before receiving drugs. Due to this added time, about seven percent of the participants had no symptoms by the time ivermectin arrived.
Despite these negative findings for ivermectin, there is still some evidence that may demonstrate that ivermectin can be useful in treating COVID-19.
In the abstract, the authors concluded that taking ivermectin had “a posterior probability of benefit of .91,” this is another way of writing that ivermectin had a 91 percent probability of being more beneficial than placebo.
The percent of probability is below 95 percent, making the benefit of ivermectin insignificant.
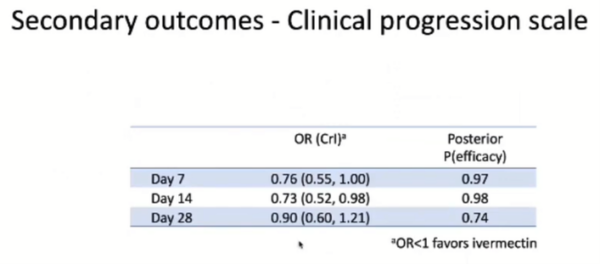
Another secondary endpoint showed that by day 14, ivermectin already had a statistically significant 27 percent benefit with 98 percent probability of efficacy.
The FDA and NIH did not respond for comments by press time.







0 Comments:
Post a Comment